Abstract
Background
Diffuse large B-cell lymphoma (DLBCL) is the most frequent subtype of HIV-associated lymphoma. Since the introduction of combined antiretroviral therapy (cART), the prognosis of HIV-related DLBCL has substantially improved, resembling that of the general population. However, non-Hodgkin lymphoma still remains the first cause of AIDS-related deaths.
The International Prognostic Index (IPI) is the most widely used score for DLBCL and it has been validated in the rituximab era (R-IPI). However, it has limited accuracy to identify a very high-risk prognostic subset. Although IPI has been demonstrated to be useful for predicting prognosis in HIV-related DLBCL, new scores subsequently developed, such as National Cancer Comprehensive Network IPI (NCCN-IPI), GELTAMO-IPI and a new score, which includes data from peripheral blood count, have not been applied in the HIV setting. The aim of this study was to assess the prognostic significance of the new variables -beta2-microglobulin (β2M), lymphocyte/monocyte (L/M) ratio and red blood cell width (RDW)- and to validate the new scores in a series of homogeneously treated HIV-related DLBCL patients.
Methods
Retrospective multicentric study of patients with HIV infection diagnosed with DLBCL in 16 hospitals from GELTAMO group in Spain, from 1998 to 2020. All patients were treated with R-CHOP and cART +/- radiotherapy. The main clinical and biological variables were collected. Peripheral absolute neutrophil, lymphocyte and monocyte counts were studied, including L/M ratio and CD4 + lymphocyte count. Moreover, HIV load, serum lactate dehydrogenase (LDH), β2M and RDW were evaluated. Univariable and multivariable analysis were performed using the binary logistic regression model for complete response (CR) rate and Cox proportional-hazards regression model for overall survival (OS) and progression-free survival (PFS). Survival curves were plotted by the Kaplan-Meier method and compared by the log-rank test. The discrimination power of IPI, aaIPI (age-adjusted IPI), R-IPI, NCCN-IPI, GELTAMO-IPI and the new score including L/M ratio (L Bento et al., Br J Haematol. 2020) was assessed by the C-index.
Results
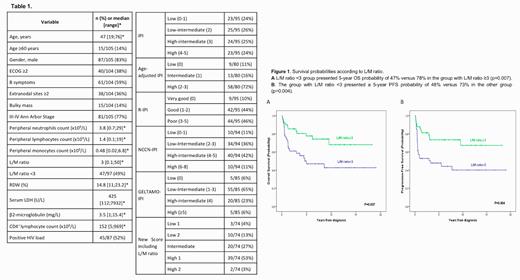
One hundred and five patients were retrospectively analysed with a median follow up of 7.08 (0.36-25.21) years. The characteristics of the patients are summarized in Table 1. In the univariable analysis, performance status ≥2, extranodal sites ≥2, lymphocytopenia and low L/M ratio were associated with shorter OS and shorter PFS probabilities. Neutropenia was also associated with lower OS and advanced Ann Arbor stage was associated with lower PFS. On the other hand, monocytosis, low CD4 + lymphocyte count, positive HIV load and high values of serum LDH, RDW and β2M had no prognostic impact. By multivariable analysis, only L/M ratio <3 emerged as an unfavourable prognostic factor for OS and PFS, with harzard ratios (HR) (95%CI) of 2.515 (1.256;5.039) and 2.563 (1.314;5), respectively (Figure 1).
With the aim of validating the prognostic power of each score system, the patients were divided in two groups: patients corresponding with low or intermediate-low risk versus those with intermediate-high or high risk. R-IPI, NCCN-IPI and the new score including L/M ratio showed significant differences in two groups for CR rate, OS and PFS. IPI also significantly discriminated the groups for PFS. NCCN-IPI was the strongest score to discriminate OS with a C-index of 0.638, and the new score including L/M ratio was the best one for CR rate and PFS discrimination, with a C-index of 0.669 and 0.666 respectively.
Conclusions
Lymphocyte/monocyte ratio is a strong prognostic factor, which can be used in patients with DLBCL and HIV infection. NCCN-IPI and the new score including L/M ratio provided the best discriminative capacity to predict prognosis in patients with HIV-related DLBCL treated with R-CHOP and cART.
Supported in part by Gilead Sciences S.L., Spain (GLD19/00121); 2017 SGR288 (GRE) from CERCA Programme/Generalitat de Catalunya, and by funds from Josep Carreras International Foundation and "la Caixa" Foundation.
Lopez-Guillermo: Roche, Gilead/Kite, Celgene, Novartis, Janssen, AbbVie, Spectrum: Consultancy, Honoraria, Research Funding. Salar: Janssen: Consultancy, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Gilead: Research Funding. de la Cruz Vicente: Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Eusapharma: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Ferrer Lores: Janssen: Membership on an entity's Board of Directors or advisory committees. Abrisqueta: Janssen: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Roche: Consultancy, Honoraria; BMS: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria. Sancho: Roche, Janssen, Celgene-BMS, Gilead, Novartis, Takeda: Honoraria, Speakers Bureau; Roche, Janssen, Celgene-BMS, Gilead, Novartis, Incyte, Beigene: Speakers Bureau. Ribera: SHIRE: Consultancy, Speakers Bureau; ARIAD: Consultancy, Research Funding, Speakers Bureau; TAKEDA: Consultancy, Research Funding, Speakers Bureau; NOVARTIS: Consultancy, Speakers Bureau; AMGEN: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Research Funding, Speakers Bureau. Navarro: NOVARTIS, Roche: Honoraria; EUSA Pharma: Consultancy; GILEAD, EUSA Pharma: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal